Last week QuantuMDx achieved yet another milestone by introducing Laboratory Information System (LIS) connectivity to the Q-POC™ software.
The latest software release for the Q-POC™ platform, 3.2, is a significant upgrade that enables test records to be automatically transferred to a LIS via Wi-Fi or ethernet local area network connection.
But what are the benefits of adding LIS communication functionality to a rapid, point of care molecular diagnostic platform?

Patient safety and security
Laboratories and other sites of testing are busy workspaces, never more so than during the COVID-19 pandemic. Now, more than ever, ensuring test results are quickly, accurately, and securely transferred to a patient’s medical records, so they can be reviewed and reported by their doctor, is a critical aspect of a high-quality healthcare system.
Reporting an incorrect test result due to human error or significant delays in test result reporting could have severe negative impacts on the health of those being tested or others who interact with them.
Increased productivity
Human resources in many health care sectors are under an ever-increasing strain. Increased demand for services, longer operating hours, increased staff absences due to illness, and a lack of qualified staff – all make it more important than ever to introduce automation where it is safe and efficient to do so.
Entering test result into a centralised patient record can be a time-consuming process and can introduce mistakes due to human error. By connecting the Q-POC™ platform directly to an LIS or middleware host, test records are automatically transmitted to the patient’s centralised records, allowing the staff to focus on running tests, not entering information into digital forms.
Improved quality management
Quality management is an essential component of the modern clinical laboratory and is a core focus of the International Standards Organisations (ISO) 15189 standard for medical laboratories1. Part of a well-functioning Quality Management System is monitoring key performance metrics and taking actions to correct or improve on these metrics. Key metrics in the Diagnostics Laboratory include the “time to report results” and the “reporting performance” of those results.
The Q-POC™ software release 3.2 is designed with a “set it and forget it” approach. Once connected with an LIS or middleware host, patient test records, in the Health Level 7 (HL7) v3 Clinical Document Architecture (CDA) standard, are automatically and securely transferred to patient health records. Reporting times, reporting accuracy and efficiency of result reporting are positively impacted with instantaneous test record transfer.
Want to find out more?
Life is better in so many ways when we can diagnose infectious diseases at the point of need. It’s immediate, easier and more efficient with the Q-POC™ platform. Discover it now.
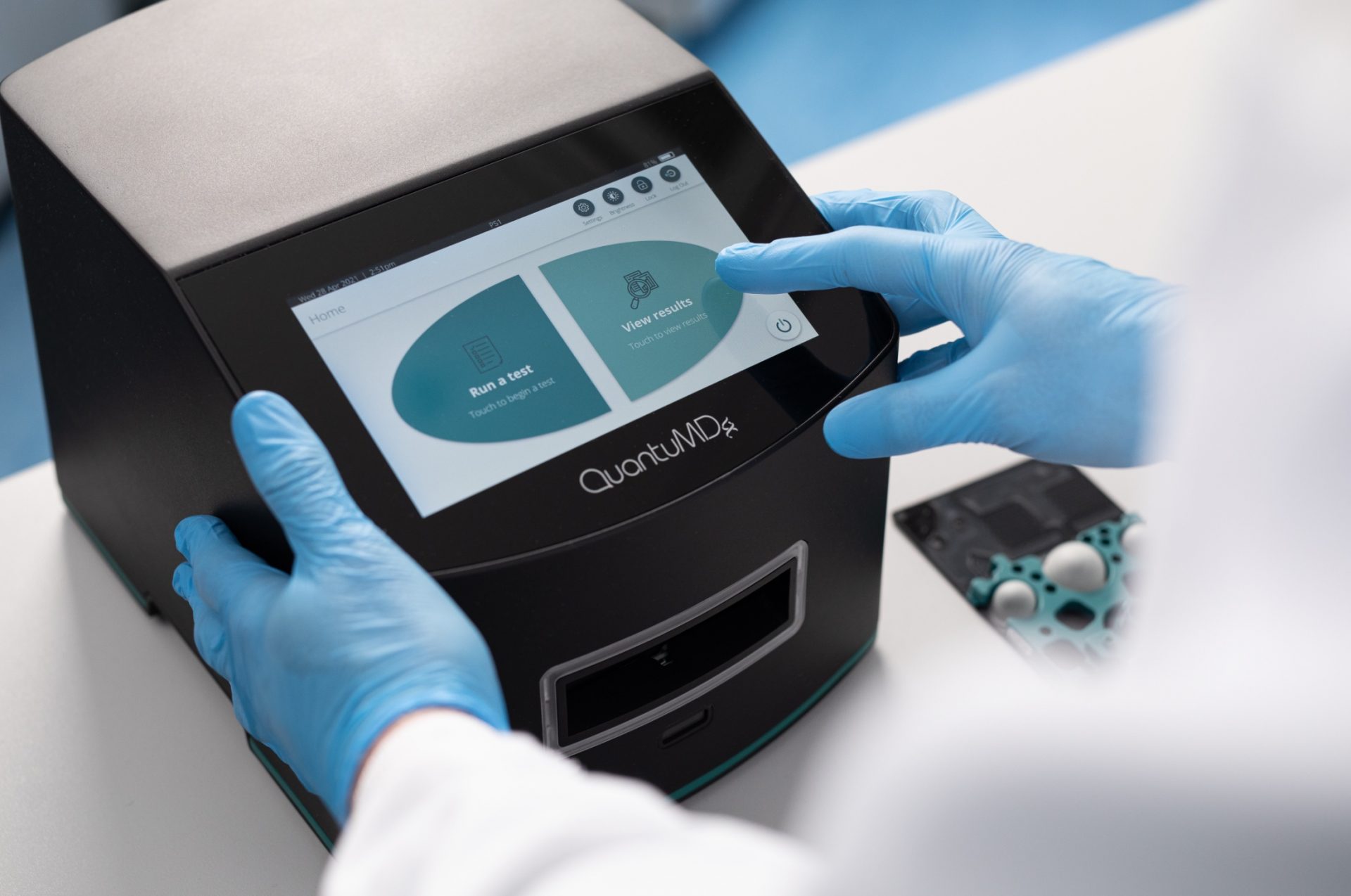
Q-POC™ platform
A rapid multiplex PCR testing system that delivers results in approximately thirty minutes at the point of need.








