The Role of Innovative Diagnostics
Sunday 24 March 2024 is World Tuberculosis Day.
March 24 is a day dedicated to raising awareness of tuberculosis (TB) and coincides with the date in 1882 when Robert Koch first described his work isolating Mycobacterium tuberculosis as the causative agent of TB to the Society of Physiology in Berlin.
As we commemorate this milestone, the role of innovative diagnostics remains pivotal in the ongoing battle against TB.
Through advancements in diagnostic technologies like our TB-Host Immune Response Assay (TB-HIRA) and CAPTURE-XT®, we at QuantuMDx are working to transform TB management by enabling earlier detection, more accurate diagnosis, and prompt initiation of treatment.
Tuberculosis Milestones
Since 1882, there have been many advances in our understanding of TB disease, diagnosis, treatment and prevention. Here are some highlights (ref: TBAlert):

Tuberculosis remains a global concern
Despite these advances, TB remains a worldwide public health crisis.
TB is the second leading infectious disease after COVID-19; an estimated 10.6 million people became ill with TB and 1.3 million people died from TB in 2022.
Multidrug-resistant TB (MDR-TB, meaning M. tuberculosis that is resistant to three or more of the drugs available for treatment) is a particular worry; 5.2% of cases are caused by MDR-TB and not many people with drug-resistant TB (only about 2 in 5) accessed treatment in 2022.
Existing TB diagnostics
TB represents a spectrum of disease, ranging from a cleared TB infection, to TB infection that is under control by the immune system, to incipient and subclinical disease stages, and finally, active TB disease.
Different diagnostic methods are needed to diagnose TB occurring at different stages. Most tests focus on the diagnosis of active TB, whereas methods to detect TB infection (previously known as latent TB) and the prediction of progression to active TB are limited.
Tests available for the detection of TB infection, current relying on the tuberculin skin test (TST) and interferon-gamma (IFN-γ) release assays (IGRA) and these tests can only tell us so much of the TB story. Once positive, they remain positive and offer no other diagnostic information.
Advancing TB diagnostics
There is an urgent unmet clinical need for rapid and accurate TB diagnostic tests suitable for the spectrum of disease.
Advances in TB diagnostics would contribute significantly to the health target of the United Nations Sustainable Development Goals: to end the TB epidemic by 2030.
These should include:
1) improved identification methods for infected individuals who are more likely to progress to active disease
2) non-sputum-based testing
3) testing solutions for patient groups who have limited access to healthcare services.
Innovative TB diagnostics
Section 7 of the WHO Tuberculosis Global Report 2022 provides a comprehensive summary of innovative technologies in the TB diagnostic pipeline.
Most technologies in development are molecular tests for the detection of TB disease and/or drug resistance detection. Biomarker-based assays for TB disease detection using samples other than sputum are also well-represented.
The pipeline lists several improved interferon-gamma release assays (IGRAs) for TB infection detection and three M. tuberculosis antigen-based skin tests that are more specific than the current TST.
Aerosol capture technologies for TB disease detection and culture-free, targeted-sequencing solutions for detection of TB drug resistance are being investigated.
This is also the case for computer-aided detection (CAD) for digital chest radiography and other artificial-intelligence (AI)-based tools, such as a digital stethoscope.
Innovating TB diagnostics at QuantuMDx
Our mission is to develop innovative TB diagnostics that are suitable for use in any setting regardless of healthcare infrastructure, so that TB can be detected and treated sooner.
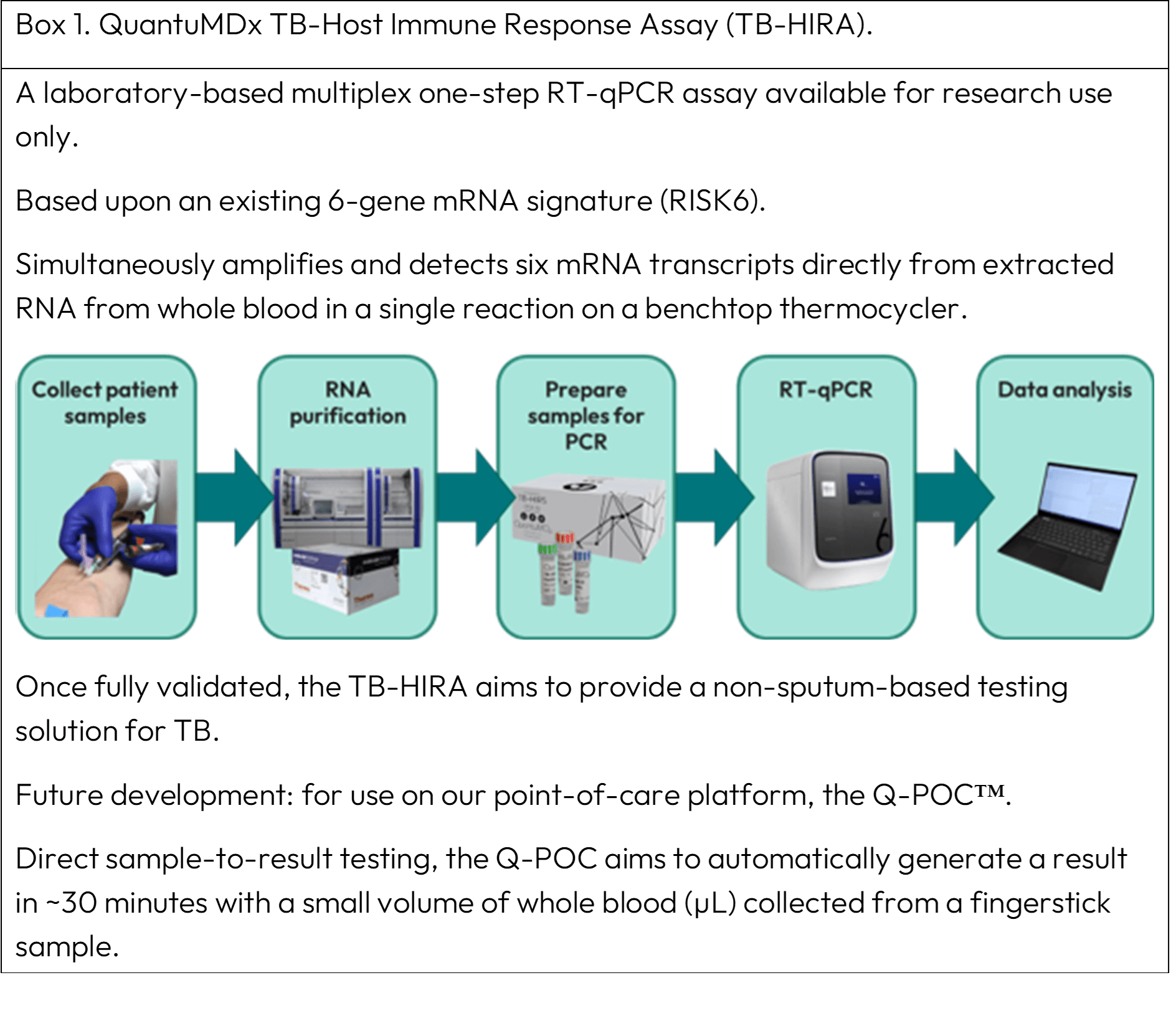
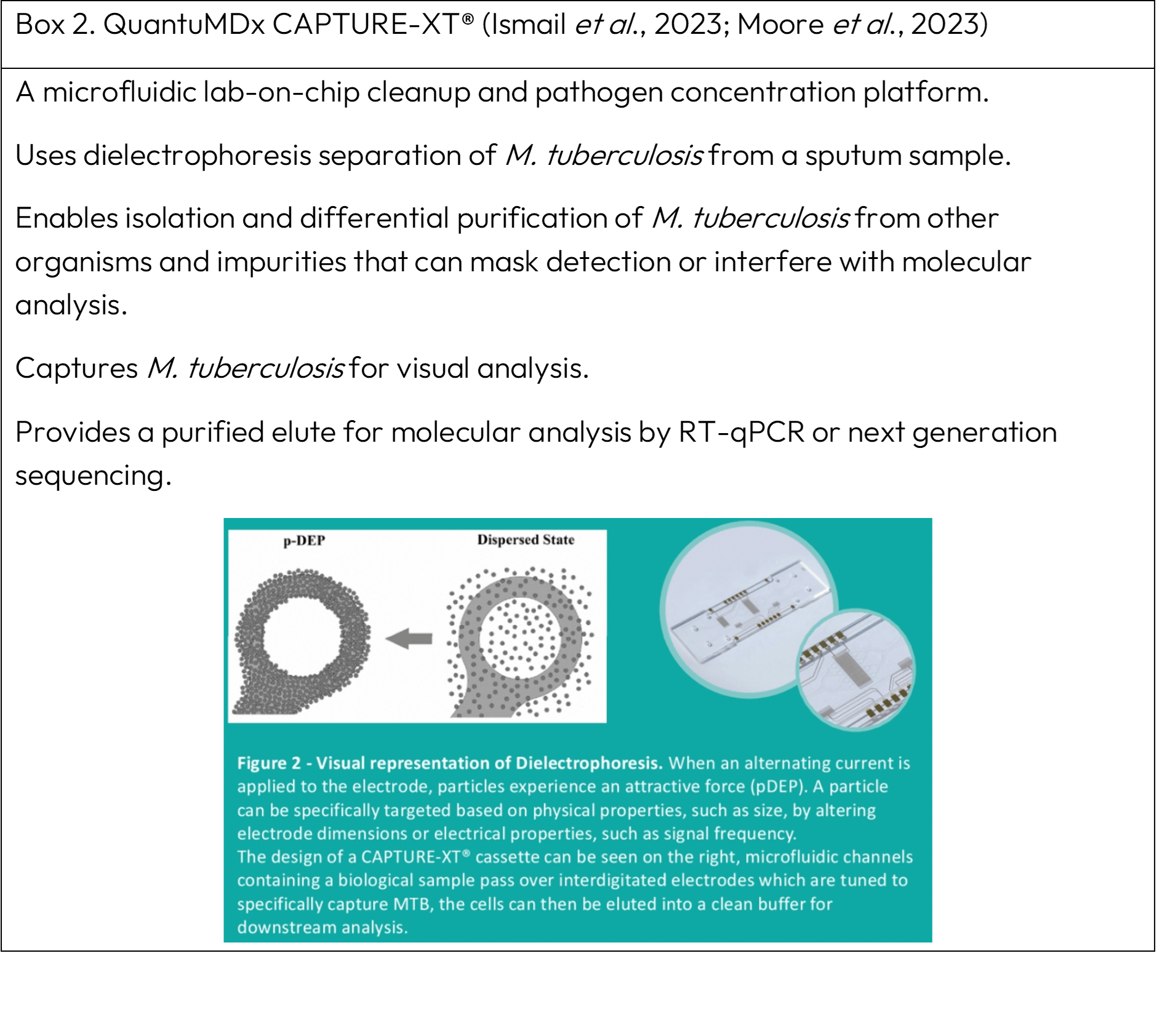
Boxes 1 and 2 describe two innovative technologies, a TB-Host Immune Response Assay (TB-HIRA) and CAPTURE-XT® that are currently under development.
TB-HIRA
TB-HIRA represents a paradigm shift in TB diagnosis, harnessing the interplay between the host immune system and M. tuberculosis, offering rapid and accurate identification of TB from a whole blood sample.
CAPTURE-XT®
CAPTURE-XT® enhances TB detection through improved bacterial capture. By concentrating M. tuberculosis bacterial cells from sputum, CAPTURE-XT® facilitates detection, thereby enabling proactive clinical intervention.
We need you!
In the spirit of World TB Day, and the collaborative need to accelerate global efforts to end TB, we invite you to get in touch with the QuantuMDx team.
If you would like to find out more about the potential of CAPTURE-XT applications at the point-of-care, would like to collaborate to evaluate TB-HIRA in clinical studies or be an early adopter of the TB-HIRA on the Q-POC™, we would love to hear from you.