Throughout human history, infectious diseases have been a major cause of suffering and mortality. Remarkably, it was once suggested that half of all humans who have ever lived have been killed by malaria [1].
Equally incredibly, this may represent the tip of the iceberg, as there are many other infectious diseases afflicting humans today, including respiratory infections (such as COVID-19) and sexually-transmitted infections (such as gonorrhoea).
Invariably, the earlier a patient is diagnosed with an illness, the greater the chance that they will be treated successfully. Here, we will learn about rapid, ‘point-of-care’ molecular diagnostics, describe the numerous advantages that they offer, and discuss what the future holds for infectious disease diagnosis.
‘Point-of-Care’ Diagnostic Tests
What is a rapid, ‘point-of-care’ diagnostic test?
A simple definition of a point-of-care test (POCT) is “a medical test that is conducted at or near the site of patient care” [2]. This can be expanded in many different ways.
Regardless of the exact definition, a good POCT should allow a biological specimen – such as blood, saliva, urine, or a swab specimen – to be collected and analysed at the patient’s side. An accurate test result should be returned as rapidly, accurately and inexpensively as possible. Ideally, very little specialist training should be required to:
(a) collect the specimen,
(b) use the diagnostic test, and
(c) interpret the result.
What sorts of POCT are available?
The COVID-19 pandemic has significantly increased public awareness of POCTs. It has highlighted the advantages of the two most common forms of molecular diagnostic:
- Antigen-based tests (such as lateral-flow tests, or ‘LFTs’)
- Nucleic acid-based tests (such as polymerase chain reaction (PCR) tests).
However, the pandemic has also exposed the current limitations of both approaches.
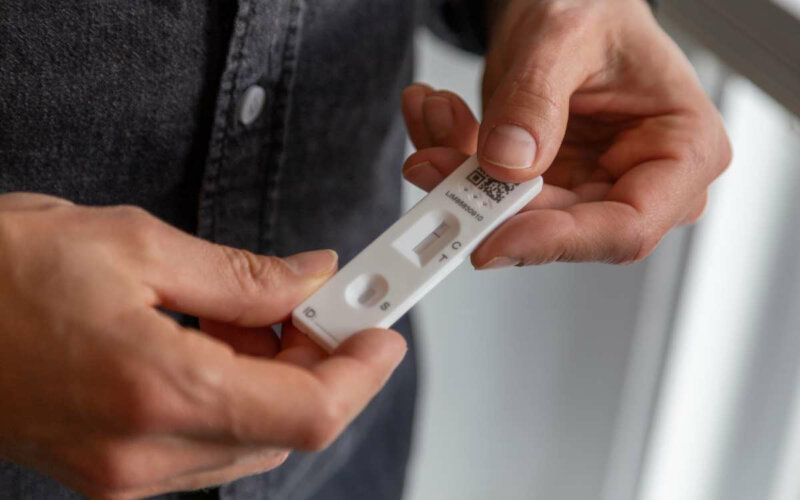
The advantages of lateral flow tests
It is likely that many reading this text will have used a COVID-19 LFT at some point in the last few years.
LFTs are usually small cassettes containing a strip of cellulose paper that holds antibodies with a high affinity for viral or bacterial antigens (such as proteins) at a particular place on the strip. The binding of specific viral or bacterial antigens to these antibodies is often represented by a colour change on the cassette.
LFTs have many advantages, including low cost, portability, and ease of use. A ‘Positive’ or ‘Negative’ test result is returned extremely quickly (typically within 15 minutes) and is usually easy for the user to understand and interpret.
The limitations of lateral flow tests
However, LFTs also have their drawbacks.
Two common disadvantages are test sensitivity (the ability to correctly identify an individual who has an infection, even when the individual has a low viral or bacterial load) and test specificity (the ability to correctly identify an individual without the infection).
It is possible for patients in the early stages of an infection – or with unusually low levels of bacteria or virus in their system – to return a ‘False Negative’ result, as there is not enough target antigen in the sample for the infection to be detectable via a LFT.
Furthermore, many LFTs are known to return ‘False Positive’ results due to antibodies cross-reacting with non-pathogen antigens.
It is also possible for patients who have recovered from an infection to continue to return ‘False Positive’ results for some time, even though they are no longer contagious.
The importance of PCR testing
PCR testing involves the amplification and detection of DNA or RNA targets. By using target-specific oligonucleotide ‘primers’, it is possible to achieve an extremely high specificity.
In addition, the amplification of the target (which is not possible in antigen-based tests such as LFTs) makes PCR testing highly sensitive. Indeed, it is common practice for clinicians to confirm a positive LFT result with a PCR test.
Infectious Disease Diagnosis at the Point of Care
Globally, the demand for high-quality POCTs is increasing, with the molecular diagnostics market projected to increase from around $2 billion in 2023 to around $3.4 billion in 2028 [3].
This is partly due to the increased incidence of infectious diseases. However, it is also due to the improvements being made in POCT development.
A shift towards PCR testing?
It is possible that PCR-based POCTs would be more widely-used if not for traditional obstacles such as:
- high cost,
- low portability,
- long turnaround times, and
- high power requirements.
These challenges are particularly difficult to overcome in low- and middle-income countries (LMICs), as well as remote regions of high-income countries (HICs).
Furthermore, expert training has often been required to use PCR-based devices and interpret the final results. However, many emerging technologies are overcoming these obstacles and making point-of-care PCR testing a reality.
The importance of early detection and fast turnaround times
Clearly, if we are to minimise suffering and limit the spread of infectious diseases, it is vital to diagnose patients as early as possible. This will improve the odds of a patient making a full recovery.
It will also enable them to sequester themselves from the rest of the population to ensure that they do not pass on the infection to others.
If a rapid, specific & sensitive POCT is available, it is possible to diagnose a patient in the very early stages of an infection; this could even be before the patient becomes symptomatic or becomes infectious to others.
A quick turnaround time becomes even more important in LMICs, where it is common for patients to not return to a centralised clinic for a diagnostic test result. This is often due to geographical distance or loss of vital family income through missing another day’s work.
It is easy to see why taking a POCT to the patient’s side and providing an accurate diagnosis in a single meeting can be highly beneficial.
The importance of identifying antimicrobial resistance
As well as diagnosing an infectious disease, it is becoming more and more important to ‘characterise’ a disease and tailor any treatment to the individual patient. The emergence of antimicrobial resistance in bacteria – including the causative agents of tuberculosis (TB) and gonorrhoea – can render many antibiotics useless.
The spread of antimicrobial resistance is being accelerated by the overuse of unsuitable or unrequired antibiotics. For example, if a clinician is unsure whether a patient’s symptoms are being caused by a bacterial or viral infection, they may recommend a course of antibiotics anyway, ‘just to be safe’.
Antimicrobial resistance is conferred at the genetic level. Hence, PCR analysis of a patient specimen can verify that the infection is caused by bacteria AND can allow antimicrobial resistance to be identified, meaning that the patient can be treated appropriately.
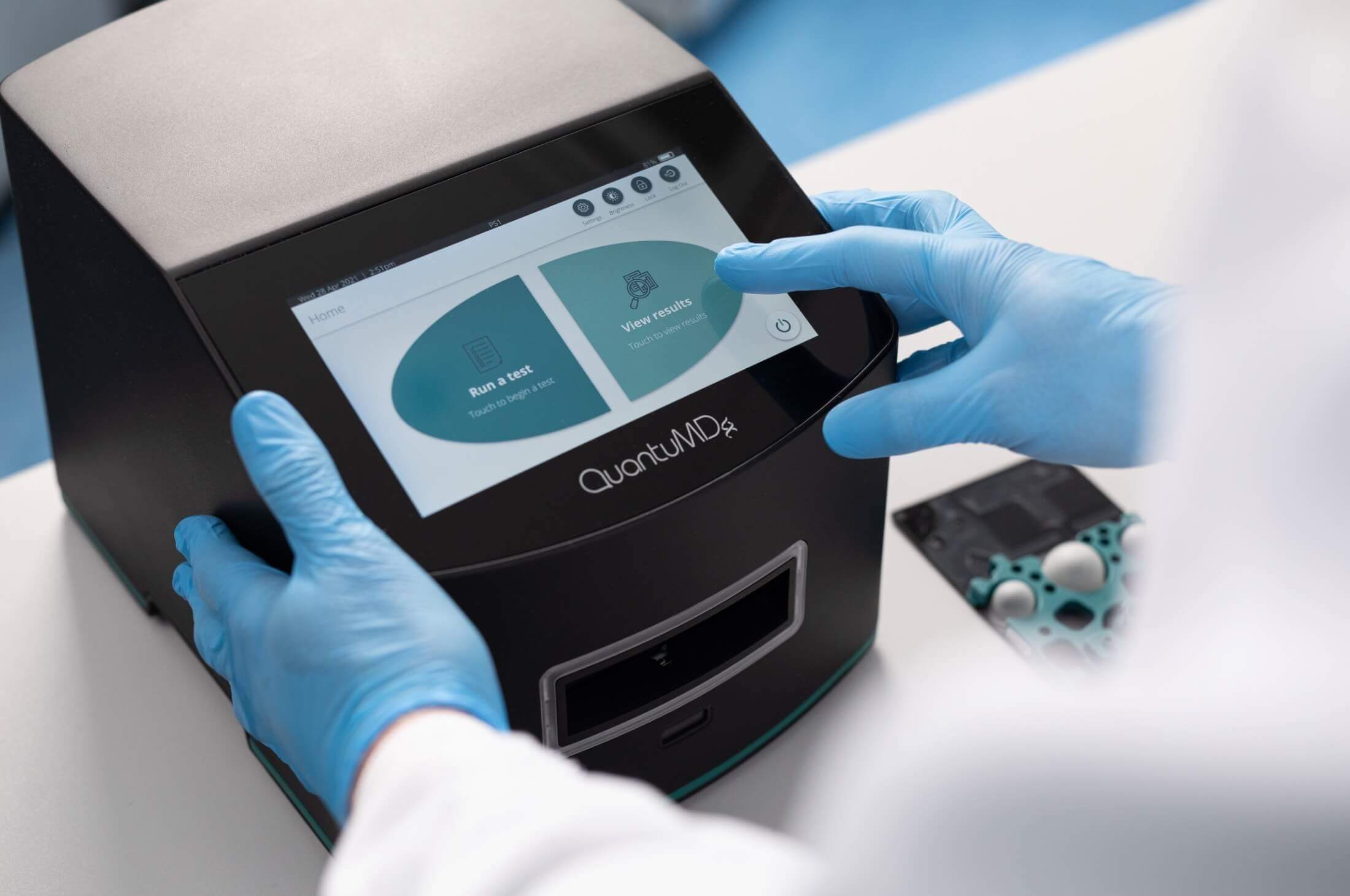
QuantuMDx: a provider of high-quality molecular diagnostics
Since the company was founded in 2008, QuantuMDx has focussed on developing high quality, affordable & rapid molecular diagnostics.
QMDx technology incorporates PCR-based testing into our portable instrument, the Q-POC™. The Q-POC™ SARS-CoV-2, Flu A/B & RSV Assay is now commercially available and delivers rapid diagnostic results in approximately 35 minutes.
In this assay, a microfluidic PCR system assay allows SARS-CoV-2, Flu A, Flu B and RSV infections to be rapidly detected and distinguished from each other. Results are provided to the user via a simple interface, without the need for any clinical expertise or data interpretation.
QuantuMDx is currently expanding its product portfolio to include many more molecular diagnostic tests.
References
| [1] | J. Whitfield, “Portrait of a serial killer,” Nature, pp. 021001-6, 03 October 2002. |
| [2] | G. Kost, “Connectivity. The millennium challenge for point-of-care testing,” Arch Pathol Lab Med, vol. 124, no. 8, pp. 1108-10, 2000. |
| [3] | “Global POC Molecular Diagnostics Market Driven by Increasing Demand for Rapid and Accurate Testing,” LabMedica International staff writers, pp. https://www.labmedica.com/industry-news/articles/294797621/global-poc-molecular-diagnostics-market-driven-by-increasing-demand-for-rapid-and-accurate-testing.html, 20 June 2023. |
.