SARS-CoV-2, the causative agent of COVID-19, and influenza are two well-known respiratory viruses, but what do you know about RSV (Respiratory Syncytial Virus)? Here, Carolyne Horner, Medical Scientific Liaison, describes aspects of RSV acute respiratory infection (RSV-ARI) and how the landscape of RSV infection has changed in recent years. An alternative approach to diagnostic testing for RSV-ARI is discussed that may help to make the best use of already stretched healthcare resources.
What’s in a name?
Syncytial (pronounced sin-SISH-uhl) is not a word common to everyday conversation and originates from the Greek language: ‘syn’ meaning together and ‘cyt’ from the word for biological cell (cyto).
When RSV was discovered in 1956, cell culture was a popular laboratory-based method for investigating how viruses interacted with living cells. When RSV was added to a layer of cells in the laboratory (known as in vitro cell culture), infected cells fused together cell membranes of neighbouring uninfected cells and the cells combined to form ‘giant cells’ or syncytia (Fig. 1).
Clinical Significance
RSV is a significant pathogen of young children, the elderly, those with comorbidities, and immunocompromised individuals.
RSV has a predisposition for infecting respiratory epithelial cells and multiples in the nasopharynx. Typically manifesting as a cough and cold, with or without a temperature. Ear infections and wheeze are common symptoms in children. Infections are usually mild and self-limiting, limited to the upper respiratory tract.
Occasionally, RSV infection may progress to the lower respiratory tract to cause bronchiolitis [inflammation of the bronchioles, tiny branching airways deep within the lungs] or pneumonia. When bronchioles become inflamed and congested with cellular debris, as a result of the respiratory infection, the already small diameter of the airways is reduced even further, resulting in difficulty breathing and lack of oxygen.
At-risk patient populations
Premature babies, neonates, and young children are at risk of RSV bronchiolitis due to the nature of their immature immune system and developing airways (the diameter of a bronchiole in a child is approximately half of that of an adult). According to the UK Health Security Agency (UKHSA, formerly Public Health England), children are most vulnerable to RSV in the first 12 months of life; up to 60% children will have been infected by their first birthday and >80% infected by the time they are two years old.
Immunity to RSV is short-lived and reinfection is common throughout life. A healthy adult with no comorbidities is likely to be infected with RSV and be non-the-wiser, apart from the discomfort and inconvenience of a common cold. The elderly, however, are at risk of more severe disease due to decreasing immunity, frequency of comorbidities, and general wear and tear of the airways.
Diagnosis, management, and treatment
For most patients infected with RSV, microbiological testing or healthcare intervention is not required; however, for those in at-risk groups and those requiring hospitalisation, respiratory samples may be tested to increase confidence in a diagnosis, support decisions associated with antibiotic prescribing, and reassure parents of young children.
In patients requiring hospitalisation, management of RSV infection is usually supportive, focusing on supplementary oxygen and feeding in young children. While ribavirin is the only antiviral drug licensed to treat RSV in severely unwell immunocompromised children, it is not recommended for routine clinical use due to lack of evidence supporting clinical benefit and toxicity concerns. Currently, there is no vaccine for the prevention of RSV infection; however, for those children at high risk of severe infection, prophylaxis using the monoclonal antibody Palivizumab is an option.
Infection Prevention and Control
RSV is a highly transmissible infectious agent spread by respiratory droplets and anything that has been in contact with these droplets; viral particles can survive on hard surfaces such as light switches, telephone keypads/handsets, and door handles for several hours. The key to reducing transmission of RSV is good hand and respiratory hygiene.
In hospitals, patients with respiratory infections, such as COVID, Flu, or RSV, may be separated from other patients in isolation rooms or cohort bays to help prevent the spread of infection to other vulnerable patients. As such, patient triage according to causative agent can be useful to allocate beds appropriately and reduce the likelihood of nosocomial transmission.
RSV has a significant burden on healthcare
RSV-ARI has a substantial disease burden in different patient groups:
| In children <5 years | |
| Hospital admission rate (per 1,000 children/year) | Premature babies: 63.85 <1 year: 19.19 <5 years: 4.37 |
| Case fatality rate (per 1,000 children) | <1 year: 6.60 <5 years: 6.21 |
| In older adults (≥65 years) without any comorbidities | |
| Annual incidence rate (per 1000 persons/year) | 6.7 |
| Hospital admission rate (per 1000 persons/year) | Developed countries: 1.0 Developing countries: 0.3 |
| Case fatality rate (per 1000 persons) | Developed countries: 1.6% Developing countries: 9.1 % |
| In older adults (≥65 years) with a comorbidity* | |
| Annual incidence rate (per 1000 persons/year) | 30.3 |
| Hospital admission rate (per 1000 persons/year in adults ≥65 years with CHF or COPD) | 13.2 |
| Case fatality rate (per 1000 adults with any comorbidity) | 11% |
The number of RSV-attributable GP appointments, hospitalisations, deaths and antibiotics prescribed per average RSV season in England are shown in Table 1.
Costs associated with RSV infection are staggering. In 2017, the global cost of RSV-ARI management (inpatient and outpatient) in children aged <5 years was estimated to be €4.82 billion. The average cost per episode of inpatient management of RSV-ARI without follow-up was estimated to be €3452 and €299 for outpatient management, increasing to €8591 and €2191 respectively, when patients required follow-up for two years.
Recent estimates of the average direct costs per non-hospitalised episode of RSV in older adults from Europe ranged from €11.74 from a patient perspective; to €14.62 from a healthcare provider perspective, €26.37 per episode from a healthcare payer perspective, reaching €30.75 from a societal perspective (mean total cost per episode).
A changing respiratory landscape
Given the huge burden of RSV alone (i.e., not including influenza and COVID-19) on healthcare in terms of primary care consultations, hospitalisations, and deaths, it is important for healthcare providers to be able to plan for the winter respiratory season, when the anticipated need of their services will be greatest. Operational planning has become more problematic following the emergence of SARS-CoV-2.
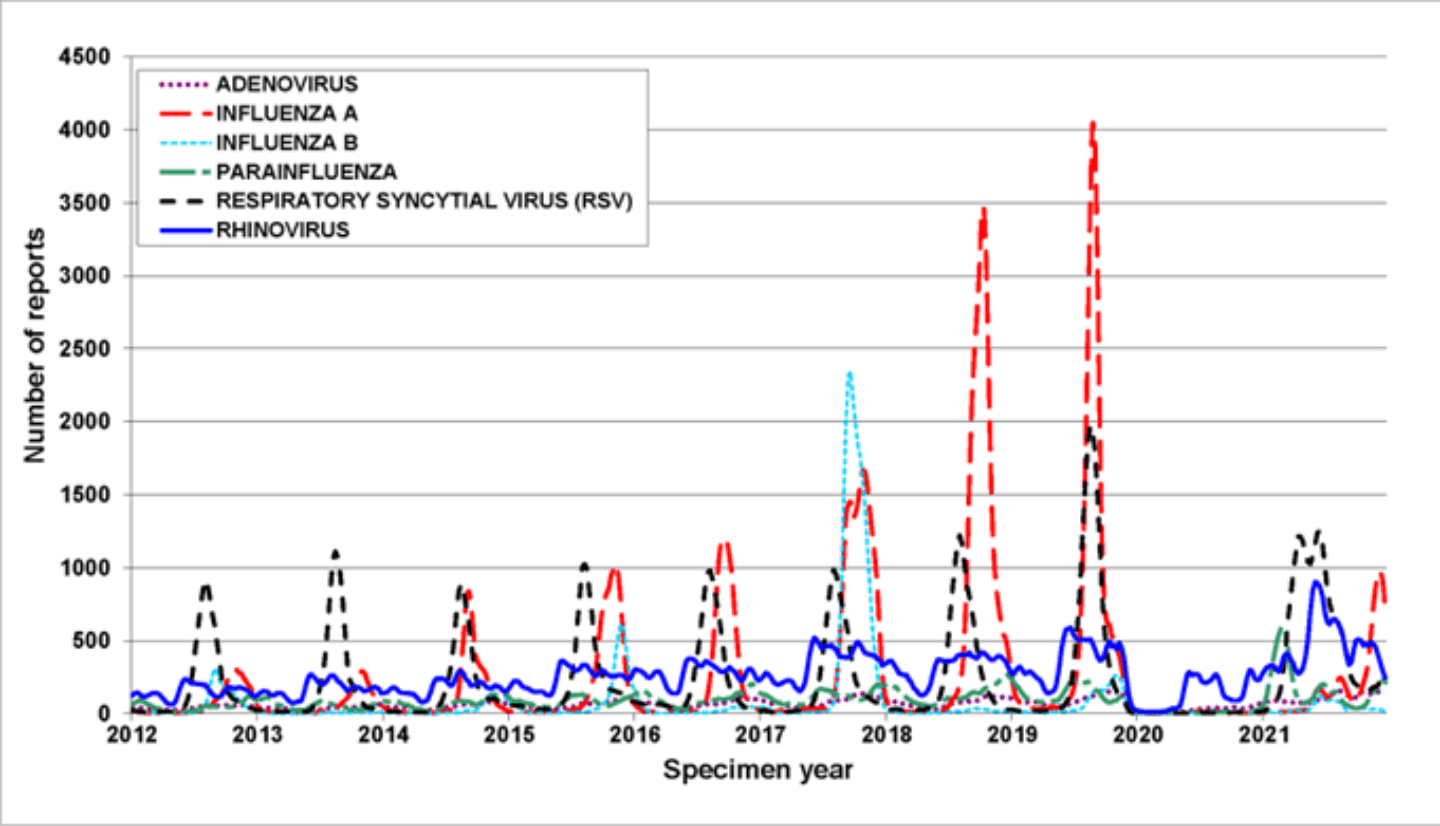
Before the COVID-19 pandemic, the seasonality of RSV was highly predictable. Typically, in temperate regions RSV had a sharp peak during the winter months (February in the northern hemisphere and July in the southern hemisphere), whereas tropical regions had less of a peak and encountered RSV at raised levels over several months (February-July in both hemispheres). Since the emergence of SARS-CoV-2 as a dominant respiratory virus, the ‘respiratory season’ as we know it has changed markedly.
Winter respiratory seasons of RSV in children under 5 years old in England proceeded as expected from 2015/16 to 2019/20, with peak RSV activity typically occurring around weeks 47-50 (November-December), with a mean 8697 cases per season. In stark contrast, winter season 2020/21, when SARS-CoV-2 was circulating, had no peak of RSV activity and only 47 cases were reported. Unexpectedly, prolonged RSV activity occurred ‘out of season’ in weeks 11-39 2021 (March-September), when 12,150 cases were reported compared with 895 cases that were predicted. Similar unseasonal patterns of RSV activity have been reported in other countries, such as Australia, Japan, Spain. This change in predictability of peak respiratory virus infections is not unique to RSV (Fig. 2).
Implications of an unpredictable RSV-ARI season
The global change in ‘non-typical respiratory seasons’ is a serious concern and has “implications for clinical service demand, testing strategies, and timing of palivizumab RSV prophylaxis.”
As we enter winter season 2022/23, more people are likely to be susceptible to RSV infection owing to a decrease in natural immunity due to lower levels of RSV activity in recent years. Natural immunity is particularly important in pregnant women as maternal antibodies offer protection to neonates.
A susceptible population and an increase in RSV activity could mean a higher number of cases, a proportion of which will be severe, meaning there may be more people than usual requiring hospitalization for respiratory infections. Of course, RSV infections are only one of a multitude of other factors that need to be considered to forecast service demand. Lower-than-usual influenza activity, increasing energy costs, unknown severity of weather conditions, and healthcare systems that have not fully recovered from the disruption of the pandemic are other factors for consideration.
Importance of an accurate diagnosis
RSV, SARS-CoV-2, influenza A and B viruses are of particular concern compared with the multitude of other respiratory viruses because of an association with lower respiratory tract infections and severity of infection, leading to a possible need for healthcare intervention and/or hospitalisation. RSV, SARS-CoV-2 and influenza require different patient management strategies in terms of (Table 2):
- Availability of antiviral options
- Supportive therapeutic options
- Infection prevention and control isolation procedures
- Public health interventions, disease notification and outbreak management
- Vaccine availability
Clinical differentiation of RSV, SARS-CoV-2, influenza is likely to be inaccurate given the overlapping clinical signs and symptoms (Table 2). In clinical situations when microbiological confirmation of a respiratory tract infection is required, respiratory samples are usually sent to a centralised laboratory for testing. The nature of centralised testing (e.g., transportation and batching of samples, separate RNA extraction and amplification steps) leads to an inevitable delay in results being available to make real-time clinical decisions.
A different approach
Advances in molecular technologies mean that there are more diagnostic options available for accurate diagnosis of acute respiratory infections in the locations that need them most. Platforms that can be used at the point-of-need have the potential to offer results in real-time of a patient consultation. In turn, they enable real-time patient management, discussions about therapeutic and treatment options as well as more timely interventions, in terms of patient triage, infection prevention and control or public health.
The benefits of testing patients for respiratory tract infections at the point-of-need are manyfold, including:
- Facilitate patient-focused clinical pathways
- Guide appropriate antibiotic prescribing for bacterial infections and reduce antimicrobial resistance (AMR)
- Appropriate triage of patients who need hospitalization
- Decrease nosocomial transmission by reducing the length of time patients are waiting in assessment cohort areas
- Optimum use of healthcare resources, such as isolation rooms, personal protective equipment (PPE) and nursing time
Summary
For most, RSV infection is a mild and self-limiting infection of the upper respiratory tract, not usually associated with any lasting side effects. Certain patient groups, however, are at increased risk of severe RSV disease, often requiring hospitalisation and supportive therapy. The number of RSV-attributable appointments in primary care, hospitalisations, deaths, and antibiotics prescribed per average RSV season are considerable and the burden of RSV disease globally is substantial. Since the emergence of SARS-CoV-2, RSV activity has been unpredictable leading to a larger population of susceptible individuals and making forecasting for service demand difficult.
Curious to know more?
The Q-POC™ platform is a rapid multiplex PCR testing system that delivers fast, accurate and actionable results in approximately 30 minutes at the point of need. The Q-POC™ SARS-CoV-2 Flu A/B RSV Assay, a single-use disposable test cassette, detects Orf1, N and S genes of SARS-CoV-2, the matrix protein of Flu A, the non-structural protein of Flu B, and the nucleoprotein and matrix protein of RSV genomic RNA, from nasal mid-turbinate, or nose and throat swabs taken from individuals suspected of having an acute respiratory infection.
Figures
Figure 1. A simplified diagram to depict the formation of syncytia in RSV-infected cells in vitro. (Modified from Domachowske and Rosenberg, 1999.)

Following infection: [A] Day 1, cells (depicted by the pattern filled area) appear healthy; [B] Day 3, many cells have fused together to form ‘giant’ cells or syncytia (unpatterned, irregular-shaped forms); Day 5, most of the cells are dead and only scraps of cells remain.
Figure 2. Circulation of six common respiratory viruses in England and Wales, reported from PHE and NHS laboratories (Week 18 2012 – Week 16 2022). The green arrow indicates the start of the COVID-19 pandemic.
Tables
Table 1. RSV-attributable burden of GP episodes, hospitalisations, deaths, and antibiotics prescribed in England per average RSV season (1995-2009).
| Taylor et al., 2016 | Fleming et al., 2015 | |
| Population (years) | 0-17 | 18+ |
| GP episodes (n) | 450,158 | 487,247 |
| Hospitalisations (n) | 29,160 | 17,799 |
| Deaths (n) | 83 | 8482 |
| Antibiotics prescribed (n) | 416,133 | 916,036 |
Table 2. Comparison of RSV, Influenza A/B and SARS-CoV-2.
| Name | Characteristic | RSV | Influenza A/B | SARS-CoV-2 |
| Discovery | Year | 1956 | 1930s | 2019 |
| Taxonomy | Family | Paramyxoviridae | Orthomyxoviridae | Coronaviridae |
| Subfamily | Pneumovirinae | – | – | |
| Genus | Pneumovirus | Influenzavirus A/B | Coronavirus | |
| Viral particle | Shape | Pleomorphic | Spherical | Spherical |
| Enveloped | Yes | Yes | Yes | |
| Genome | Genetic Material | RNA | RNA | RNA |
| Organisation | Linear | Linear | Linear | |
| Single-stranded | Yes | Yes | Yes | |
| Sense | Negative | Negative | Positive | |
| Segmented | No | Yes | No | |
| Size (approx.) | 15kb | 13.5kb (A) 14.5kb (B) | 30kb | |
| Infection | Incubation (days) | 3-6 | 2-3 | 3-5 |
| Symptom onset | Gradual | Sudden | Gradual | |
| Typical* symptoms Upper respiratory | Rhinitis (runny nose, sneezing, nasal congestion) | ü | ü | ü |
| Cough | ü | ü | ü | |
| Sore throat | û | ü | ü | |
| Loss of taste/smell | û | û | ü | |
| Lower respiratory | Wheezing | ü | û | û |
| Difficulty breathing | ü | û | ü | |
| Systemic | Fever | ü | ü | ü |
| Malaise | ü | ü | ü | |
| Complications | Bacterial superinfection | Uncommon | Common | Uncommon |
| Long COVID | No | No | Yes | |
| Reinfection | Common | Uncommon | Common | |
| Management | Antiviral | Ribavirin | Oseltamivir Zanamivir | Nirmatrelvir/Ritonavir Remdesivir Molnupiravir |
| Antibiotics | NO! | NO! | NO! | |
| Corticosteroids | No | No | Yes# | |
| Oxygen | Yes | Yes | Yes | |
| Prevention | Natural immunity | Short-lived | Longer | Short-lived |
| Vaccine available | No | Yes | Yes | |
| Public Health | Notifiable | No | Yes | Yes |








